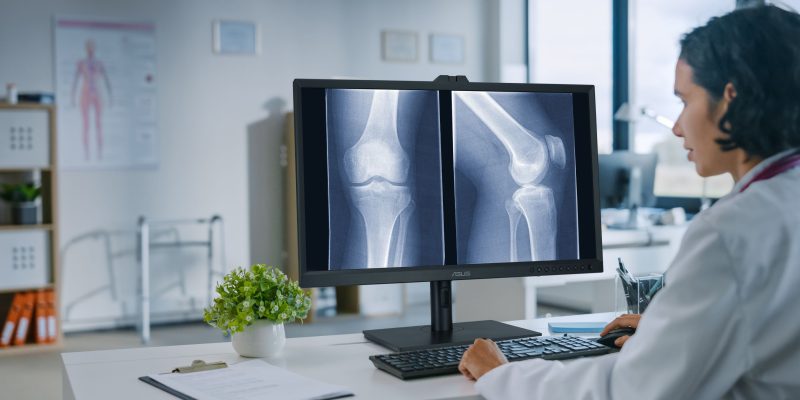
ASUS MH Series Clinical Displays Achieve FDA Class 1 Certification
TLDR
- ASUS MH Series Clinical Displays Attain Class 1 FDA Certification
ASUS proudly announces that the latest additions to its MH series clinical displays have been designated as Class 1 Devices by the US Food and Drug Administration (FDA).
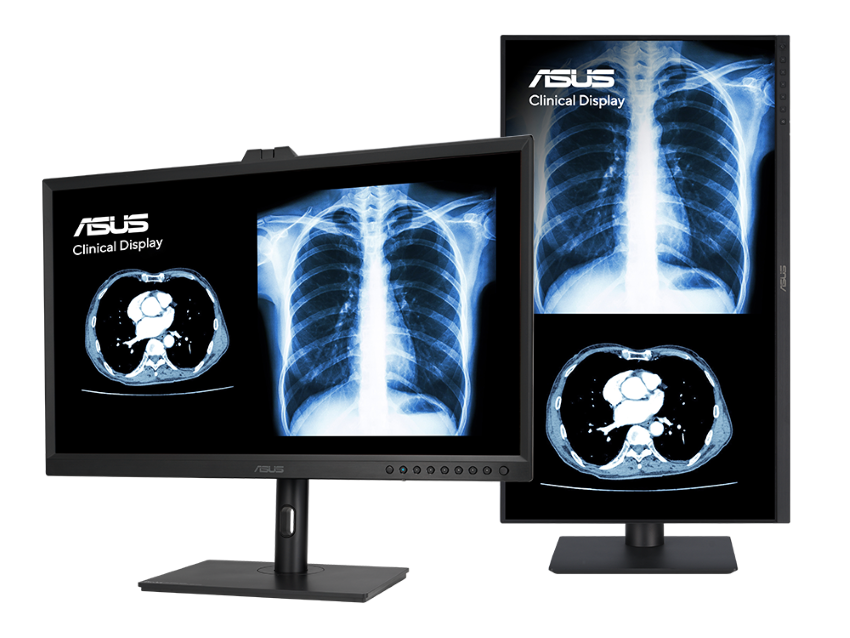
The models include the 32-inch 8-megapixel MH3281A, the 27-inch 4-megapixel MH2741A, and the 23.8-inch MH2441A. Primarily designed for radiology and Picture Archiving and Communication System (PACS) use, these monitors ensure precision and reliability in medical imaging.
Striving for Excellence in Medical Imaging
All three monitors adhere to the DICOM Part 14 Grayscale Standard Display Function (GSDF) AAPM TG270 standard. Equipped with features such as scheduled calibration, dynamic brightness compensation, and dynamic DICOM compensation, they guarantee consistent and highly accurate medical images.
The monitors also boast an anti-glare, low-reflection (AGLR) coating on the panel, ensuring optimal visibility. Rigorous performance tests, TÜV Rheinland certifications for flicker-free performance and low blue light emissions, and compliance with ISO 13485 requirements validate their suitability for use in clinics and large medical institutions.
Global Compliance and Expansion Efforts
Beyond FDA certification, ASUS aims to secure CE marks, indicating conformity with EU Medical Device Regulation guidelines. Simultaneously, approval from the Taiwan Food and Drug Administration (TFDA) is being sought for these clinical displays.
Actively engaging with major medical institutions globally, ASUS is committed to understanding industry needs and adapting to the evolving medical-device market, providing innovative solutions for the European and Asian markets.










